Dylan has finished his MChem project entitled “Visualisation of Aromaticity and Antiaromaticity via the Computation of the Chemical Shielding on Multi-Dimensional Grids.” You can find his report here. The purpose of his project was to develop a convenient method for computing shielding tensors on a grid around a molecule. The developed code is available via github.
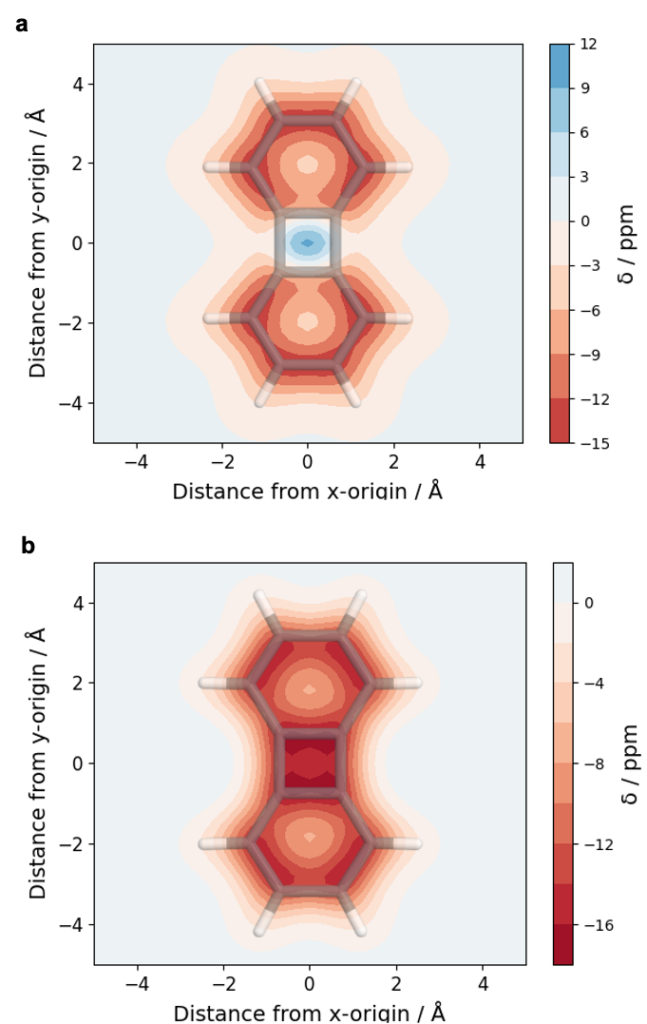
Below, an analysis of biphenylene is shown in the singlet (a) and triplet (b) state. For the singlet this representation highlights the aromaticity (red) of the benzene rings whereas the central 4-membered ring is found to be antiaromatic (blue). In the triplet (b), the whole molecule is found to be aromatic (red) according to Baird’s rule.
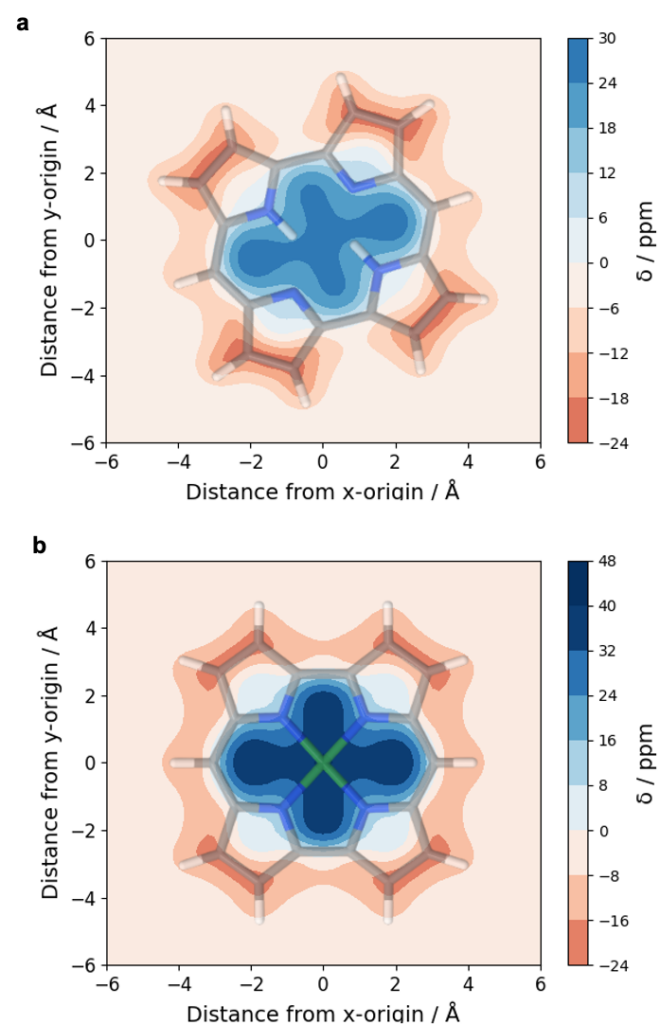
An analysis of norcorrole using either its doubly protonated form (a) or a nickel complex (b) highlights the antiaromaticity at the centre of this molecule whereas an aromatic pathway is found at the perimeter (see also [P. B. Karadakov, Org. Lett. 2020, 22, 8676]).
For more examples of how this type of analysis is used in the literature, see e.g., [Angew. Chemie – Int. Ed. 2020, 59, 19275] and [ChemPhysChem 2021, 22, 741].