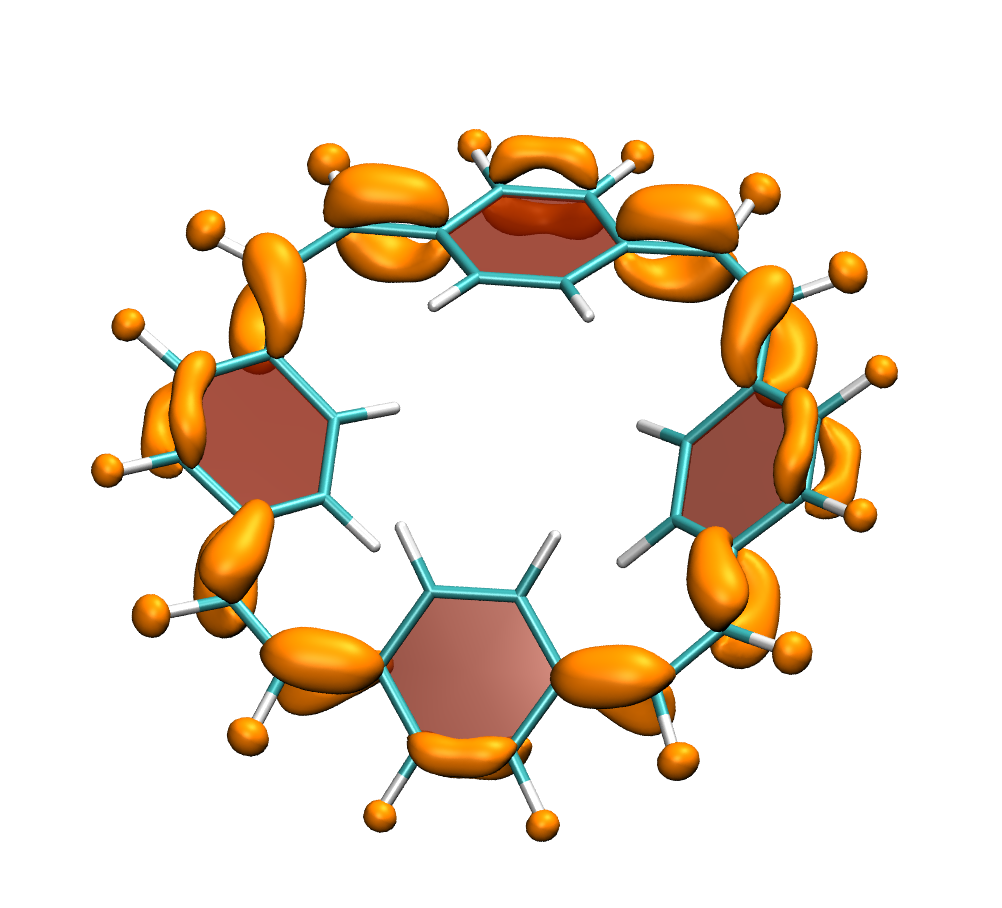
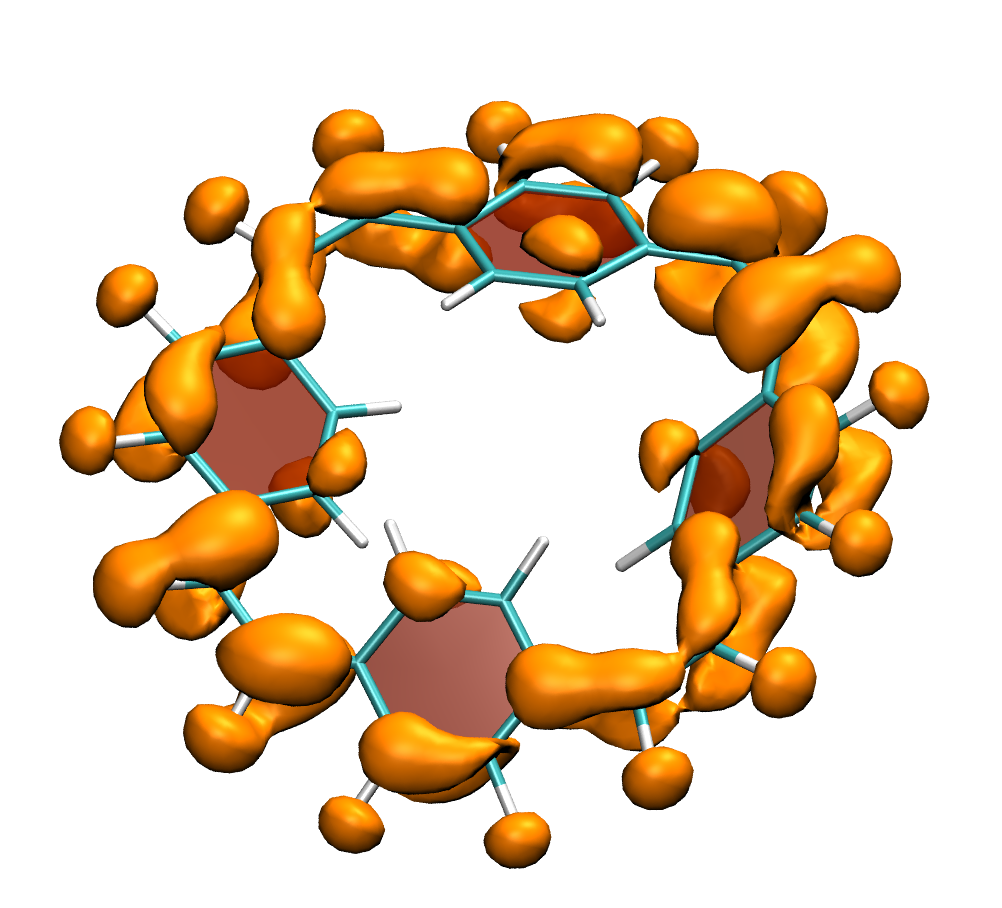
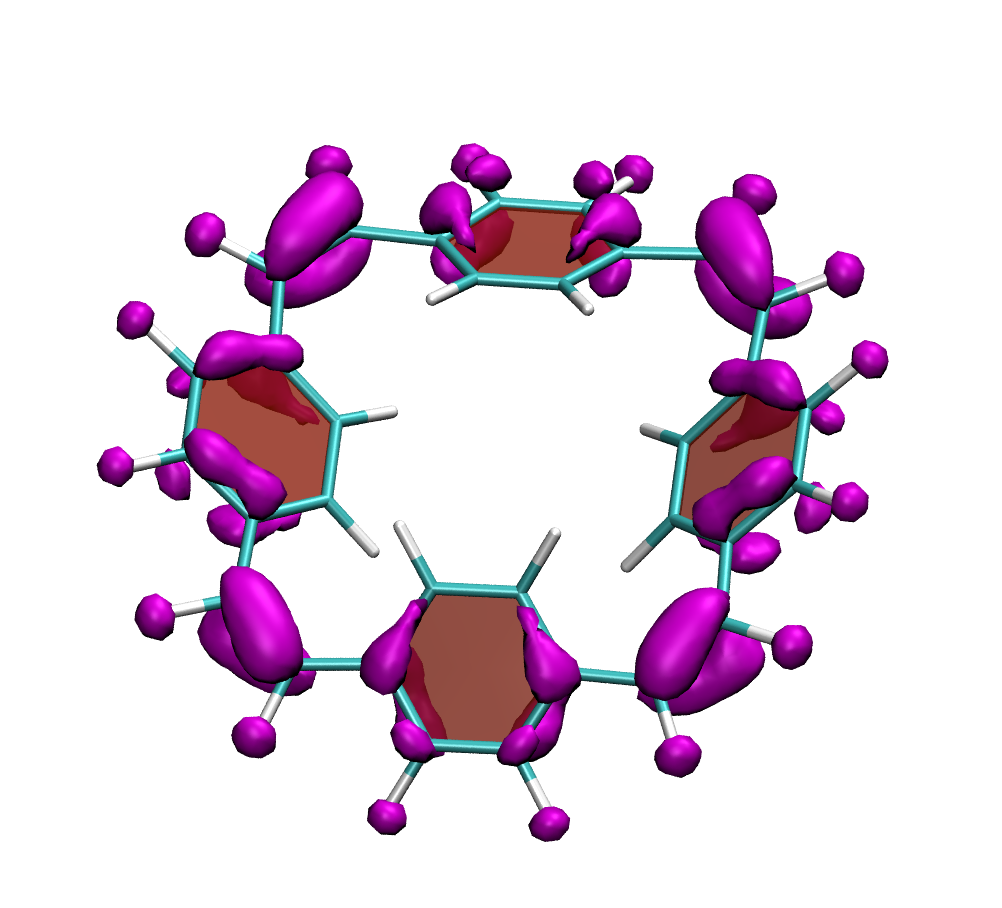
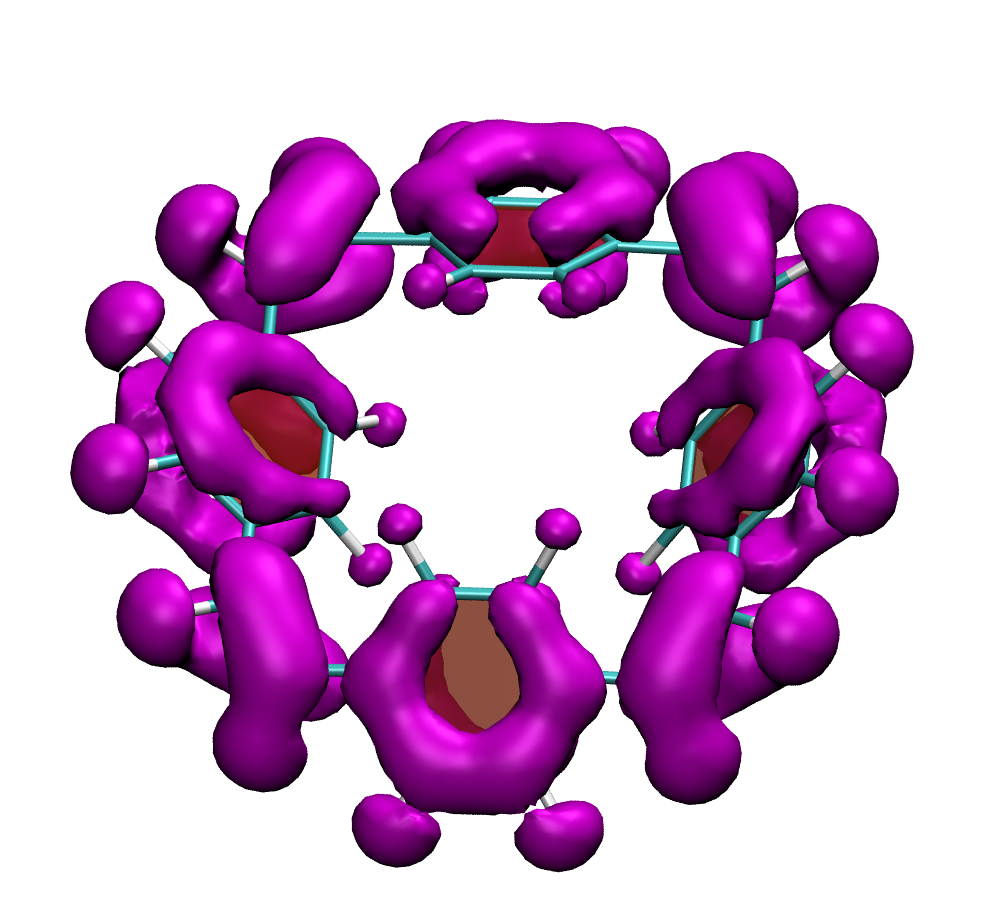
How do macrocycles with [4n] electrons behave? Are there signatures of their formal antiaromaticity and how can their properties be tuned for practical applications? A recent study, led by Florian Glöcklhofer (Imperial College, London) endeavours to tackle these questions. A set of macrocycles based on [2.2.2.2]cyclophanetetraenes was synthesised, their redox and optical properties were measured, and a detailed computational analysis was performed.
Clear signatures of the unique properties of these macrocycles was found considering their large Stokes shifts (>1.5 eV) along with the ease of producing doubly charged states. A detailed computational analysis traces these properties back to the aromaticity of the excited and doubly charged states, respectively. In addition, it is illustrated how the properties of the macrocycles can be systematically varied with introduction of functional groups and variation of the aromatic units.
The study just appeared as a preprint on ChemRxiv: Functional Group Introduction and Aromatic Unit Variation in a Set of π-Conjugated Macrocycles: Revealing the Central Role of Local and Global Aromaticity.
Below, electron density difference plots for the charged states of the parent molecule paracyclophanetetraene are shown highlighting the cyclic symmetry of the electron attachment. The 2+/2- and 6+/6- states are aromatic whereas the 4+/4- singlet states are antiaromatic.